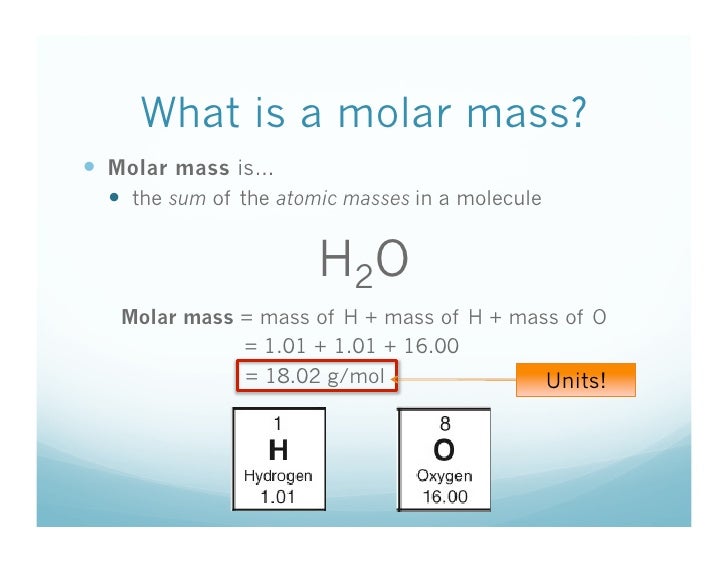
Atomic Mass Of H2o
- One mole of H 2 O is made up of 2 moles of hydrogen atoms and 1 mole of oxygen atom. Molar mass of atoms Mass of 1 mole of Hydrogen atoms= 1 g /mol Mass of 1mole of Oxygen atoms = 16 g/mol.
- Molar Mass, Molecular Weight and Elemental Composition Calculator Enter a chemical formula to calculate its molar mass and elemental composition: Molar mass of H2O is 18.01528 ± 0.00044 g/mol.
- Molecular weight of H2O or mol This compound is also known as Water or Dihydrogen Monoxide. The SI base unit for amount of substance is the mole. 1 grams H2O is equal to 0.61792 mole.
- Molecular Mass Of H20
- H2o Molar Mass
- H20 Molecule Mass
- Calculate The Atomic Mass Of H2o
- Atomic Mass Of H2o
- Atomic Mass Of 2h2o
The molar mass and molecular weight of H2O is 18.01528.
The molar mass (M) is a physical property in chemistry. It is defined as the mass of a given substance (chemical element or compound) divided by its amount of substance. The SI unit for molar mass is Kg/mol. For significant reasons, molar masses are always expressed in g/mol.
Molar mass of H2O: M (H2O) = 18g/mol
A mole can be defined as a collection of atoms whose mass is equal to the atomic mass in grams. The number of atoms in a mole is 6.02 x 1023. This number is known as Avogadro’s number.
1 mole H2O has the same number of molecules as in 18.015 g of H2O. Or one mole hydrogen molecule contains the same number of molecules as in 2.016 g of H2. The mass of one mole of a substance is known as Molar Mass.
How to calculate the molar mass of H2O?
Water is made up of hydrogen and oxygen, with a formula of H2O. There are 2 moles of Hydrogen and 1 moles of Oxygen. Therefore, Molar mass of water would be: 2 x H + O = 2 x 1.008 + 16 = 18.016 g/mol = 18 g/mol
Molar mass equation:
The volume of the liquid usually can be calculated very easily. We use the gas equation
P V = n R T
Where,
P is the pressure of the gas
V is the volume of the gas
n is the amount of substance of gas (also known as number of moles)
R is the ideal, or universal, gas constant, equal to the product of the Boltzmann constant and the Avogadro constant.
T is the temperature of the gas
After calculating the number of moles of the gas, we can use the relationship:
Moles of the liquid = Mass in grams / Molar mass
So, Molar mass can be calculated as: Molar mass = Mass in grams / Moles of the liquid
This method of finding the molar mass of a liquid is called as VICTOR- MEYER’S METHOD.
Molar mass calculator
Significance on Molar Mass
There is a difference between molecular mass and molar mass. Molar mass is never measured directly. It is calculated by summing up the atomic masses. The molecular mass is measured directly. For example, molecule of water and molecule of heavy water. The molecular masses are different as both have different isotopes of hydrogen.
It is a very useful term and is used in different chemical calculations of stoichiometry and other colligative properties. For example, in the Avogadro’s gas law equation:
PV = nRT
Where, n is number of moles.
Examples:
Molar mass of a compound is usually calculated by adding the individual molar mass of all the elements present in it.
Example 1: How to calculate the molar mass of Sodium hydroxide?
The formula of Sodium hydroxide is NaOH. It contains sodium, hydrogen and oxygen.
Let us add the molar masses of all these elements:
Na: 22.98 g/mol
O: 16 g/mol
H: 1.008 g/mol.
Adding all the atomic masses:
22.98 + 16 + 1.008 = 39.98 g/mol
The molar mass of Sodium hydroxide is:
40gram /moles (rounded of)
Example 2: Calculate the molar mass of Copper sulphate.
Copper sulphate contains: Cu + S + 4 x O
Cu = 63.55 g/mol
S = 32 g/mol
O = 16g/mol
Cu + S + 4 x O = 63.55 + 32 + 4 x 16 = 159.55 = 160 g/mol.
So, molar mass of Copper sulphate is 160 grams/mol.
Useful resources:
- Learn gas laws on Wikipedia: see the basic concepts on molar masses, you can learn the concepts from this link.
- The schematic diagram to understand the computing of the molar masses. Also, definitions are given to learn the basics of chemistry.
- Another online calculator is accessible to calculate the molar mass of any substance. You can visit this site for more practice.
25th Aug 2019 @ 32 min read
In chemistry, the molar mass is an important quantity. It measures the mass of a mole of a given substance.
Definition
The molar mass of a given substance is defined as the mass of a sample divided by the moles of that substance in the sample. In other words, it is the mass per mole of a substance.
It is usually denoted by the symbol M.
Unit
The standard unit is g mol−1. The SI unit is kg mol−1, however, it is very uncommon.
Mole
We know that one mole of a substance consists of 6.022 140 76 × 1023 elementary particles. This number (aka Avogadro’s constant) is mostly approximated to 6.022 × 1023. Thus, one mole of carbon contains 6.022 × 1023 atoms of carbon.
When we say the molar mass of carbon is 12.0 g mol−1, it means one mole of carbon weighs 12.01 g. In other words, 6.022 × 1023 atoms of carbon weigh 12.01 g.
Important of Molar Mass
In chemistry, calculations are related to chemical reactions and stoichiometry. Calculations often include quantities like molarity, molality, mole fraction, molar volume. All these involve the number of moles. Although there is no direct way to measure the number of moles of any substance, the number of moles can be calculated by knowing the molar mass of the substance. The molar mass links the mass of a substance to its moles. Thus, by knowing the molar mass, we can determine the number of moles contained in a given mass of a sample.
Let me make it more clear with an example of sodium chloride. The molar mass of sodium chloride is known; it is 58.44 g mol−1. If we have to measure one mole of sodium chloride, there is no instrument that can directly measures it. But we know 58.44 g of sodium chloride is equivalent to one mole of it. So, we measure 58.44 g of NaCl with a weight scale, which is equivalent to one of 58.44 g. Hence, we have one mole of NaCl measured.
Some of the important points regarding the molar mass are as follows:
- It is a bulk property, not atomic property, of a substance.
- It is an intensive property. So, it does not vary with the size of a sample.
- It is dependents on percentages of consistents of a sample. Thus, it can vary with a terrestrial location.
- It is used to convert the mass of a substance to its mole and vice versa.
Atomic Mass and Molar Mass
The atomic mass and the molar mass are confused with one another. But they are two different quantities with a definition. The table below describes the differences between the two.
| Atomic Mass | Molar Mass |
| The atomic mass is the sum of the mass of protons, neutrons, and electrons. | It is the mass of a mole of a substance. |
| It is denoted by ma. | The symbol used for it is M. |
| It has a unit of the unified mass unit (u) or the atomic mass unit (amu). | g mol−1 is the standard unit for the molar mass. |
| The atomic mass is an atomic property. | It is a bulk property. |
| It does not account for isotopes. | The existence of isotopes is accounted for since it is a bulk property. |
| The atomic mass is constant for a particular isotopic element. It does not vary from sample to sample. For example, the atomic mass of carbon-12 is 12 u. This is true for all atoms of carbon-12 in the universe. | It could vary from sample to sample depending on the percentages of constituents in the sample. |
Average Atomic Mass to Molar mass
If the average atomic mass (ma) or average molecular mass is known, we can convert it into the molar mass (M) with the help of the molar mass constant (Mu).
The average atomic mass is measured in the unified mass unit (u). The unified mass unit is related to the molar mass constant (Mu) by the Avogadro constant (NA).
ma is the average atomic mass or the average mass of an atom. So, the mass of a mole of the atom is ma × NA. But this quantity is in the unified mass unit (u). The above equation can convert u to g mol−1.
Therefore, the average atomic mass divided by one unified mass unit times the molar mass constant results in the molar mass.
The precise value of Mu is 0.999 999 999 65(30) g mol−1. But this value approximately equals 1 g mol−1.
From the above equation, we can say the numeric value of the molar mass and the average atomic mass is approximately equal. This is also true for molecules. The table below lists the elements (and the compounds) with the average atomic mass and the average molar mass.
Element (or Compund) | Average Atomic Mass (or Average Molecular Mass) (u) | Divide (÷) | Unified Mass Unit (u) | Multiple (×) | Molar Mass Constant (Mu) | Equal to (=) | Molar Mass (1 g mol−1) |
Hydrogen (H) | 1.008 u | ÷ | 1 u | × | 1 g mol−1 | = | 1.008 g mol−1 |
Carbon (C) | 12.011 u | ÷ | 1 u | × | 1 g mol−1 | = | 12.011 g mol−1 |
Oxygen (O) | 15.999 u | ÷ | 1 u | × | 1 g mol−1 | = | 15.999 g mol−1 |
Nitrogen (N) | 14.007 u | ÷ | 1 u | × | 1 g mol−1 | = | 14.007 g mol−1 |
Potassium (K) | 39.098 u | ÷ | 1 u | × | 1 g mol−1 | = | 39.098 g mol−1 |
Calcium (Ca) | 40.078 u | ÷ | 1 u | × | 1 g mol−1 | = | 40.078 g mol−1 |
Chlorine (Cl) | 35.45 u | ÷ | 1 u | × | 1 g mol−1 | = | 35.45 g mol−1 |
Hydrogen Gas (H2) | 2.016 u | ÷ | 1 u | × | 1 g mol−1 | = | 2.016 g mol−1 |
Water (H2O) | 18.015 u | ÷ | 1 u | × | 1 g mol−1 | = | 18.015 g mol−1 |
Methane (CH4) | 16.04 u | ÷ | 1 u | × | 1 g mol−1 | = | 16.04 g mol−1 |
Nitrogen Dioxide (NO2) | 46.006 u | ÷ | 1 u | × | 1 g mol−1 | = | 46.006 g mol−1 |
Ammonia (NH3) | 17.031 u | ÷ | 1 u | × | 1 g mol−1 | = | 17.031 g mol−1 |
Glucose (C6H12O6) | 180.156 u | ÷ | 1 u | × | 1 g mol−1 | = | 180.156 g mol−1 |
Atomic Weight to Molar Mass
The atomic weight (aka relative atomic mass) (Ar) is a dimensionless quantity having the numeric value of the average atomic mass. The average atomic mass and atomic weight are related by the following equation.
From the above two equations,
Therefore, the atomic weight times the molar mass constant results in the molar mass.
So, we can calculate the molar mass of any compound in the same way we calculate the atomic weight from its constituent elements.
Consider an example of glucose. Glucose molecular formula is C6H12O6; it has six carbons, twelve hydrogens, and six oxygens. The molar mass of glucose is the sum of the relative atomic mass of all the atoms in the molecular formula.
| Element | Atomic Weight | Molar Mass (g mol−1) |
| Carbon (C) | 12.011 | 12.011 |
| Hydrogen (H) | 1.008 | 1.008 |
| Oxygen (O) | 15.999 | 15.999 |
| Molar Mass of Glucose (C6H12O6) | 180.156 | |
Molar Mass of Mixtures
The molar mass of a mixture is determined using the mole fraction (xi).
where: Mmix is the molar mass of a mixture of n components, and xi and Mi are the mole fraction and the molar mass of ith component.
The above formula can also be expressed in terms of the mass fraction wi.
or
Consider a liquid-liquid mixture of water (H2O) and ethanol (C2H6O). Water is 20 % and ethanol, 80 %.
The molar mass of pure water is MH2O.
Similarly, for ethanol,
The molar mass of the mixture is Mmix.
Thus,
Measurement of Molar Mass
From Atomic Weight
The measurement from the atomic weight is the most reliable and precise method in comparison to others. The atomic weight is determined from the atomic mass and distribution of isotopes. The precision in the molar mass depends upon the precision in the measurement of the atomic weight, which depends on the atomic mass and the percentages of isotopes. Today, with the help of mass spectrometry, we are able to achieve high precision in the atomic mass.
The value of molar mass calculated from the atomic weight are reliable for all practical calculations.
From Vapour Density
Molecular Mass Of H20

The vapour density (ρ) is the mass of vapour to its volume. We are familiar with the ideal gas equation.
Here, P is the pressure of a gas occupying the volume V at the temperature T. n and R are the moles of the gas and the ideal gas constant.
Now, the number of moles (n) is the mass of the gas divided by the molar mass (M).
Using the above two equations,
If we are able to determine the vapour density of a sample of gas for a known pressure and temperature, we can estimate the molar mass of the vapour from the above equation.
From Freezing-Point Depression
When a non-volatile solute is added into a solvent, the freezing point of the solvent decreases. This decrease is called freezing-point depression. Freezing-point depression ∆TF = TFsolvent − TFsolution depends on the concentration (molality) of the solute and is governed by the equation below.
Here, ∆T is freezing-point depression, KF is the solvent dependent cryoscopic constant, b is the molality, and i is the van ‘Hoff factor (number of ions formed after the dissociation of the solute).
For a very dilute solution, .
By knowing ∆TF, KF, wsolute, and i, we can determine the molar mass, Msolute.
From Boiling-Point Elevation
Boiling-point elevation is the rise in the boiling point of a solvent due to the presence of a non-volatile solute. Similar to freezing-point depression, the rise in the boiling point depends upon the molality.
Here, ∆TB = Tsolution − Tsolute is boiling-point elevation, KB is the ebullioscopic constant, b is the molality, and i is the van ‘t Hoff factor.
For a very dilute solution,
By knowing ∆TB, KB, wsolute, and i, we can determine the molar mass, Msolute.
Examples and Calculations
H2o Molar Mass
Example 1: To Determine Molar Mass of Oleic Acid
Oleic acid is a odourless fatty acid having a molecular formula of C18H34O2. It has eighteen carbons, thirty-four hydrogens, and two oxygens.
| Element | Number of Atom | Molar Mass (g mol−1) | Total |
| Carbon (C) | 18 | 12.011 | 216.198 |
| Hydrogen (H) | 34 | 1.008 | 34.272 |
| Oxygen (O) | 2 | 15.999 | 31.998 |
| Molar Mass of Oleic Acid, (C18H34O2) | 282.468 g mol −1 | ||
Therefore, the molar of oleic acid is 282.468 g mol−1.
Example 2: To Determine Molar Mass of Reinecke’s Salt
Reinecke’s salt is a red crystalline salt with chromium in the centre as shown in the above figure. The chromium atom is surrounded by six nitrogens, four carbons, and four sulphur. The molecular formula is C4H12N7OCrS4.
| Element | Number of Atom | Molar Mass (g mol−1) | Total |
| Carbon (C) | 4 | 12.011 | 48.044 |
| Hydrogen (H) | 12 | 1.008 | 12.096 |
| Nitrogen (N) | 7 | 14.007 | 98.049 |
| Oxygen (O) | 1 | 15.999 | 15.999 |
| Chromium (Cr) | 1 | 51.996 | 51.996 |
| Sulphur (S) | 4 | 32.065 | 128.26 |
| Molar Mass of Reinecke’s Salt, C4H12N7OCrS4 | 354.444 g mol−1 | ||
Example 3: To Determine Molar Mass of Air
H20 Molecule Mass
Air is a mixture. It mainly consists 79 % nitrogen (N2) and (21 %) oxygen (O2). Other components are argon (Ar), carbon dioxide, (CO2), water (H2O), helium (He) etc., but these components are in small fraction and can be ignored.
The molar mass of nitrogen (N2) and oxygen (O2) are 28.014 g mol−1 and 31.998 g mol−1 respectively.
| Molecules | Fraction | Molar Mass (g mol−1) | Total |
| Nitrogen (N2) | 0.79 | 28.014 | 22.13 |
| Oxygen (O2) | 0.21 | 31.998 | 5.88 |
| Molar Mass of Air | ≈ 28 g mol−1 | ||
Therefore, the molar of air is 28 g mol−1.
Example 4: To Determine Molar Mass of Sodium Chloride Solution
Sodium chloride a common salt. It is white and odourless powder. Consider a solution of 50 % NaCl. 100 g of the solution consists of 50 g of NaCl and 50 g of H2O.
The molar mass of water is 2 × 1.008 + 15.999 = 18.015 g mol−1 and of sodium chloride is 22.99 + 35.45 = 58.44 g mol−1.

The molar of the solution is calculated as follows:
Calculate The Atomic Mass Of H2o
Thus, the molar mass of 50 % sodium chloride solution is 28 g mol−1.
Associated Articles
If you appreciate our work, consider supporting us on ❤️ patreonAtomic Mass Of H2o
.- 4
- cite
- response
Copy Article Cite
Atomic Mass Of 2h2o
